
EDUCATIONAL WORKSHOP
You could not join us at the IFCC Euromedlab/Worldlab 2023 for our Workshop about "The importance of FLC for detecting and monitoring monoclonal gammopathies"?
The recording is now available! Select the presentation you would like to access!

EDUCATIONAL WORKSHOP
You could not join us at the IFCC Euromedlab/Worldlab 2023 for our Workshop about "The importance of FLC for detecting and monitoring monoclonal gammopathies"?
The recording is now available! Select the presentation you would like to access!

Improving the diagnosis and management of
Multiple Myeloma
Discover the latest guidelines
The process for identifying patients with monoclonal gammopathies can be complex. Different algorithms exist worldwide for such testing.
In 2021, the College of American Pathology (CAP) has recently released new guidelines insisting notably on the importance of SPE and FLC testing in parallel for two main clinical applications (strong recommendation):
- for the initial detection of monoclonal immunoglobulin protein (M-protein) in all patients with suspected monoclonal gammopathies (MG)
- for the initial detection of M-protein in all patients with suspected amyloid light chain (AL) amyloidosis


Earlier, in 2014, International Myeloma Working Group (IMWG) guidelines had been published (Rajkumar V et al.), including updated criteria for the diagnosis of multiple myeloma.
In addition to the pre-existing CRAB criteria (Calcium elevation, Renal Insufficiency, Anemia, Bone disease), IMWG introduced the SLiM criteria (≥ 60% clonal bone marrow plasma cells, Light Chain Ratio ≥ 100, MRI > 1 focal lesions) as Myeloma Defining Events.
Smoldering Multiple Myeloma (SMM) is a heterogeneous clinical entity, including patients progressing to multiple myeloma and related end-organ damage. Identifying patients at that stage and assessing the risk that they progress quickly to multiple myeloma is crucial to adapt the monitoring and to select suitable candidates for investigational therapies.
In 2018, Lakshman A et al., introduced new 20-20-20 system to detect high-risk patients with SMM (HRSMM): BMPC% > 20%, M-protein > 20g/L, and iFLCr > 20. Patients with two or more of these risk factors are candidates for close monitoringa

Improving the diagnosis and management of
Multiple Myeloma
Discover the latest guidelines

The process for identifying patients with monoclonal gammopathies can be complex. Different algorithms exist worldwide for such testing.
In 2021, the College of American Pathology (CAP) has recently released new guidelines insisting notably on the importance of SPE and FLC testing in parallel for two main clinical applications (strong recommendation):
- for the initial detection of monoclonal immunoglobulin protein (M-protein) in all patients with suspected monoclonal gammopathies (MG)
- for the initial detection of M-protein in all patients with suspected amyloid light chain (AL) amyloidosis

Earlier, in 2014, International Myeloma Working Group (IMWG) guidelines had been published (Rajkumar V et al.), including updated criteria for the diagnosis of multiple myeloma.
In addition to the pre-existing CRAB criteria (Calcium elevation, Renal Insufficiency, Anemia, Bone disease), IMWG introduced the SLiM criteria (≥ 60% clonal bone marrow plasma cells, Light Chain Ratio ≥ 100, MRI > 1 focal lesions) as Myeloma Defining Events.

Smoldering Multiple Myeloma (SMM) is a heterogeneous clinical entity, including patients progressing to multiple myeloma and related end-organ damage. Identifying patients at that stage and assessing the risk that they progress quickly to multiple myeloma is crucial to adapt the monitoring and to select suitable candidates for investigational therapies.
In 2018, Lakshman A et al., introduced new 20-20-20 system to detect high-risk patients with SMM (HRSMM): BMPC% > 20%, M-protein > 20g/L, and iFLCr > 20. Patients with two or more of these risk factors are candidates for close monitoringa
The importance of FLC testing
FLC testing in serum is part of international guidelines for the aid in diagnosis and monitoring of Multiple Myeloma and AL Amyloidosis. 20 and 100 ratios are particularly important, respectively for SMM patient risk stratification and for detection of active MM.
The importance of FLC testing
FLC testing in serum is part of international guidelines for the aid in diagnosis and monitoring of Multiple Myeloma and AL Amyloidosis. 20 and 100 ratios are particularly important, respectively for SMM patient risk stratification and for detection of active MM.
A reliable and effective solution for
FLC testing
As a global leader in Multiple Myeloma diagnosis, Sebia offers a comprehensive menu, from Serum / Urine Protein Electrophoresis to Immunofixation / Immunotyping for a reliable detection and monitoring of monoclonal gammopathies. Sebia relies on leading-edge technology, including capillary electrophoresis instrumentation, to provide best-in-class solutions to its customers throughout the globe.
At a glance
A reliable and effective solution for FLC testing
As a global leader in Multiple Myeloma diagnosis, Sebia offers a comprehensive menu, from Serum / Urine Protein Electrophoresis to Immunofixation / Immunotyping for a reliable detection and monitoring of monoclonal gammopathies. Sebia relies on leading-edge technology, including capillary electrophoresis instrumentation, to provide best-in-class solutions to its customers throughout the globe.
At a glance

We were already equipped with Sebia solution for SPE and IF/IT, so it was making sense to streamline our activities, adding FLC testing. The test is flexible and can be adapted to several microplate processors. Analytical and clinical performance also reached our requirements. Lab technicians are satisfied with this solution, me too

We were already equipped with Sebia solution for SPE and IF/IT, so it was making sense to streamline our activities, adding FLC testing. The test is flexible and can be adapted to several microplate processors. Analytical and clinical performance also reached our requirements. Lab technicians are satisfied with this solution, me too.
We needed first some time to adapt as FLC values could not be compared directly with results previously obtained on other methods. But now, I'm really satisfied. We have results on time and we can also use the cut-off recommended in international guidelines, to initiate therapy when required.


We needed first some time to adapt as FLC values could not be compared directly with results previously obtained on other methods. But now, I'm really satisfied. We have results on time and we can also use the cut-off recommended in international guidelines, to initiate therapy when required.

Following the implementation of Sebia FLC offer in our hospital lab routine, we could dramatically reduce our reagent costs, by keeping the same activity. Indeed, Sebia FLC assays are associated with a lower retest rate for each patient. Such a cost-effective solution!

Following the implementation of Sebia FLC offer in our hospital lab routine, we could dramatically reduce our reagent costs, by keeping the same activity. Indeed, Sebia FLC assays are associated with a lower retest rate for each patient. Such a cost-effective solution!
Be the next satisfied adopter!
With Free Light Chain assays, Sebia is expanding its myeloma testing offer with a comprehensive and reliable solution. It leads to an improved patient management to initiate when needed the right treatment at the right time.
ELISA method flexibility and ease of use bring peace of mind, whatever laboratory testing activity is.
Be the next satisfied adopter!
With Free Light Chain assays, Sebia is expanding its myeloma testing offer with a comprehensive and reliable solution. It leads to an improved patient management to initiate when needed the right treatment at the right time.
ELISA method flexibility and ease of use bring peace of mind, whatever laboratory testing activity is.
Trust in results
Trust in results
- Jacobs JFM et al., 2017: "both Freelite and N Latex nephelometric assays substantially overestimate the concentration of monoclonal FLC in samples with high titers, which is caused by FLC polymerisation. In this study we confirm these data, and demonstrate that the Freelite monoclonal FLC concentration was consistently, and on average 12-fold, overestimated compared to quantification by electrophoresis"
- Pekar JD et al., 2019: "The analyzer SPA Plus did not report any alarm for antigen excess … In conclusion, our report shows that the new sandwich ELISA Sebia FLC is a good alternative to sFLC assessment and can avoid the problem of antigen excess"
- Van Helden J et al., 2019: "Sebia sFLC concentrations showed a good comparability to the SPE FLC"
"
- Jacobs JFM et al., 2017: "both Freelite and N Latex nephelometric assays substantially overestimate the concentration of monoclonal FLC in samples with high titers, which is caused by FLC polymerisation. In this study we confirm these data, and demonstrate that the Freelite monoclonal FLC concentration was consistently, and on average 12-fold, overestimated compared to quantification by electrophoresis"
- Pekar JD et al., 2019: "The analyzer SPA Plus did not report any alarm for antigen excess … In conclusion, our report shows that the new sandwich ELISA Sebia FLC is a good alternative to sFLC assessment and can avoid the problem of antigen excess
- Van Helden J et al., 2019: "Sebia sFLC concentrations showed a good comparability to the SPE FLC"
"

Sebia FLC assays show strong analytical performance
Sebia has conducted extensive interference studies (endogenous and exogenous interference studies), notably including analytes used for patient treatment. No interference has been shown at the tested concentrations for first-line or second-line therapies notably including dexamethasone, lenalidomide, daratumumab, melphalan, carfilzomib, isatuximab*.
*Source: data from FDA 510k K210623, November 2022
Sebia FLC assays show strong analytical performance
Sebia has conducted extensive interference studies (endogenous and exogenous interference studies), notably including analytes used for patient treatment. No interference has been shown at the tested concentrations for first-line or second-line therapies notably including dexamethasone, lenalidomide, daratumumab, melphalan, carfilzomib, isatuximab*.
*Source: data from FDA 510k K210623, November 2022

Beyond strong analytical performance, Sebia FLC assays have also high clinical performance that makes Sebia solution ideal in routine use.

Beyond strong analytical performance, Sebia FLC assays have also high clinical performance that makes Sebia solution ideal in routine use.
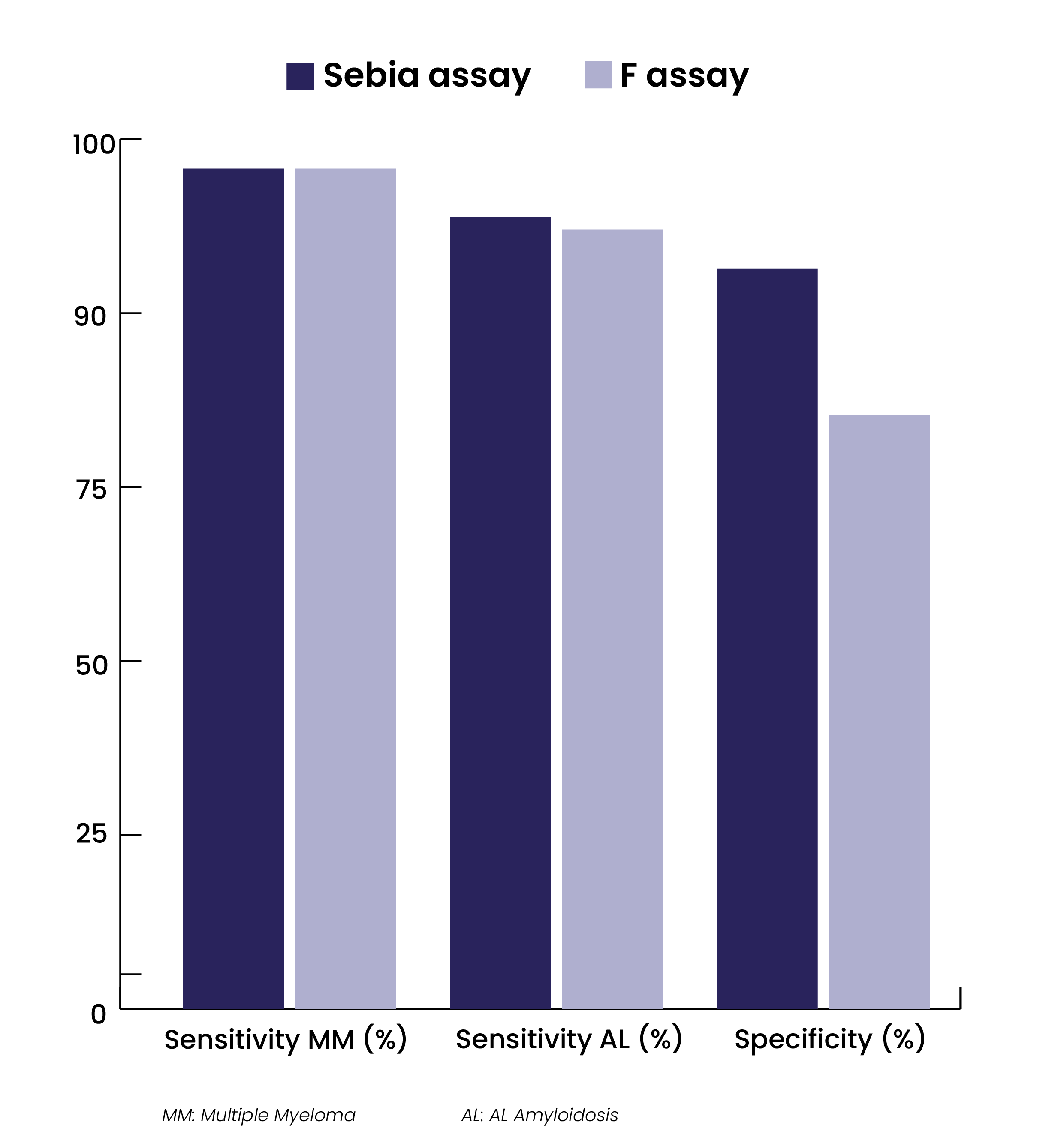
Sebia FLC assays show strong clinical performance
During the clinical evaluations performed in the US, external clinical sites have notably conducted concordance studies between Sebia and the predicate assay (F assay). Sebia FLC assays have demonstrated comparable clinical sensitivity with the predicate for the diagnosis of MM (96.6% on 177 samples from patients diagnosed with MM), better clinical sensitivity for the diagnosis of AL (91.0% on 144 samples from patients diagnosed with AL) and better clinical specificity (85.1% on 189 non-myeloma/non-amyloidosis subjects with various clinical conditions)*.
*Source: data from FDA 510k K210623, November 2022

Sebia FLC assays show strong clinical performance
During the clinical evaluations performed in the US, external clinical sites have notably conducted concordance studies between Sebia and the predicate assay (F assay). Sebia FLC assays have demonstrated comparable clinical sensitivity with the predicate for the diagnosis of MM (96.6% on 177 samples from patients diagnosed with MM), better clinical sensitivity for the diagnosis of AL (91.0% on 144 samples from patients diagnosed with AL) and better clinical specificity (85.1% on 189 non-myeloma/non-amyloidosis subjects with various clinical conditions)*.
*Source: data from FDA 510k K210623, November 2022
In addition to demonstrate strong clinical performance, "20" and "100" FLC ratio cut-points can be used with Sebia FLC assays, for an appropriate patient management.


In addition to demonstrate strong clinical performance, "20" and "100" FLC ratio cut-points can be used with Sebia FLC assays, for an appropriate patient management.
- Willrich M.A.V. et al., 2022: "Our study suggests that it is acceptable to apply the existing FLC criteria used to define MM requiring therapy and to predict SMM risk of progression when using the Sebia assay, despite the analytical differences between assays....This commutability of the iFLCr cut-points in the SMM cohort is welcome in the hematology field and provides significant information to clinicians first seeing these patients and categorizing them as having a pre-malignant or malignant PCD."
"
- Willrich M.A.V. et al., 2022: "Our study suggests that it is acceptable to apply the existing FLC criteria used to define MM requiring therapy and to predict SMM risk of progression when using the Sebia assay, despite the analytical differences between assays....This commutability of the iFLCr cut-points in the SMM cohort is welcome in the hematology field and provides significant information to clinicians first seeing these patients and categorizing them as having a pre-malignant or malignant PCD."
"
Discover more
Discover more






This section contains information intended for wide distribution and may therefore contain product details or information that is not available or valid in your country.
Please contact your local Sebia representative. Information intended for healthcare professionals. Carefully read the instructions in the reagent package inserts and instrument manuals.
